Profile: Eyenovia (NASDAQ:EYEN) is a commercial-stage ophthalmic pharmaceutical technology company.
SA Analyst Rating for EYEN: “Strong Buy”
SA Quant rating: “HOLD”
Wall Street Ratings: “Strong Buy”
Summary: Eyenovia’s APP13007 is coming up for a FDA decision next week. Will the drug win approval?
Seeking FDA Approval for APP13007 in post-surgical ocular pain and inflammation.
Type of Application: New Drug Application (SNDA).
FDA Decision (PDUFA) Date: March 4, 2024.
About the drug: APP13007 is a novel ophthalmic nanosuspension formulation of clobetasol propionate (0.05%), a potent steroid that is prescribed for skin conditions like eczema and psoriasis. APP13007’s original developer Formosa Pharma used its proprietary APNT nanoparticle to create a novel formulation of the molecule making it suitable for administration to the eye. APP13007 has a solution-like appearance offering comfort to the eyes and enhanced drug penetration into ocular tissues.

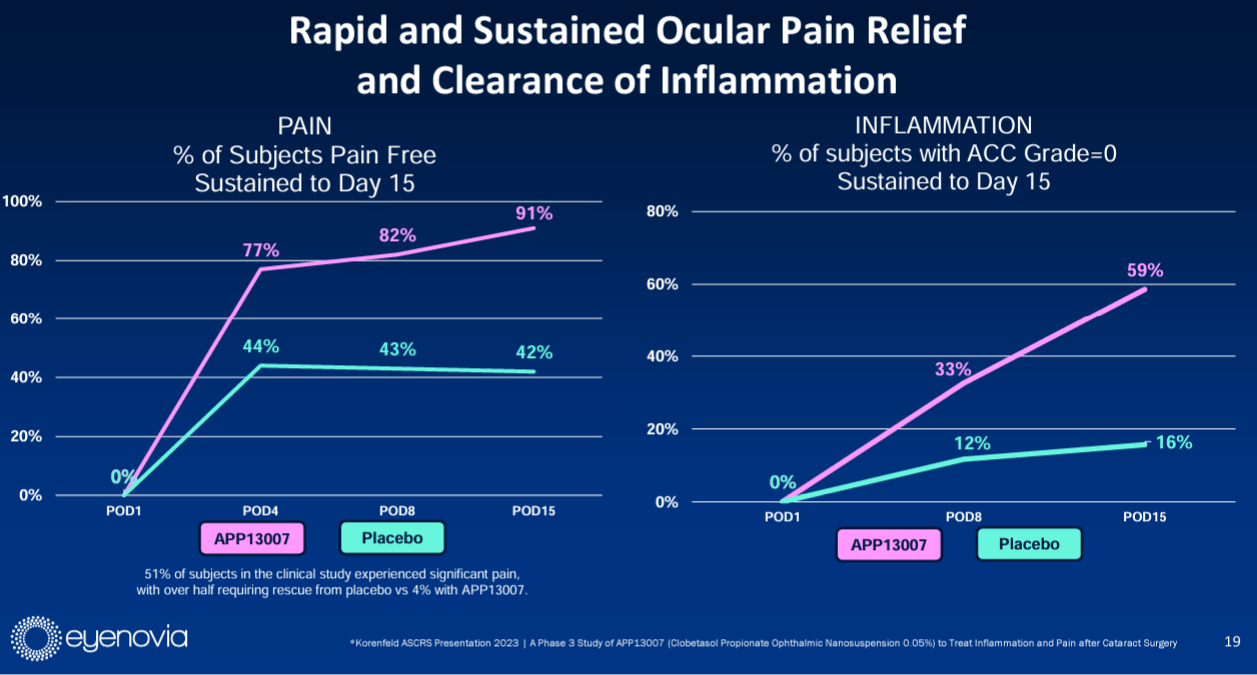
Drug’s MOA: APP13007 eyedrop applied twice daily for 14 days, showed rapid and sustained ocular pain relief and clearance of inflammation, statistically and clinically superior to placebo.
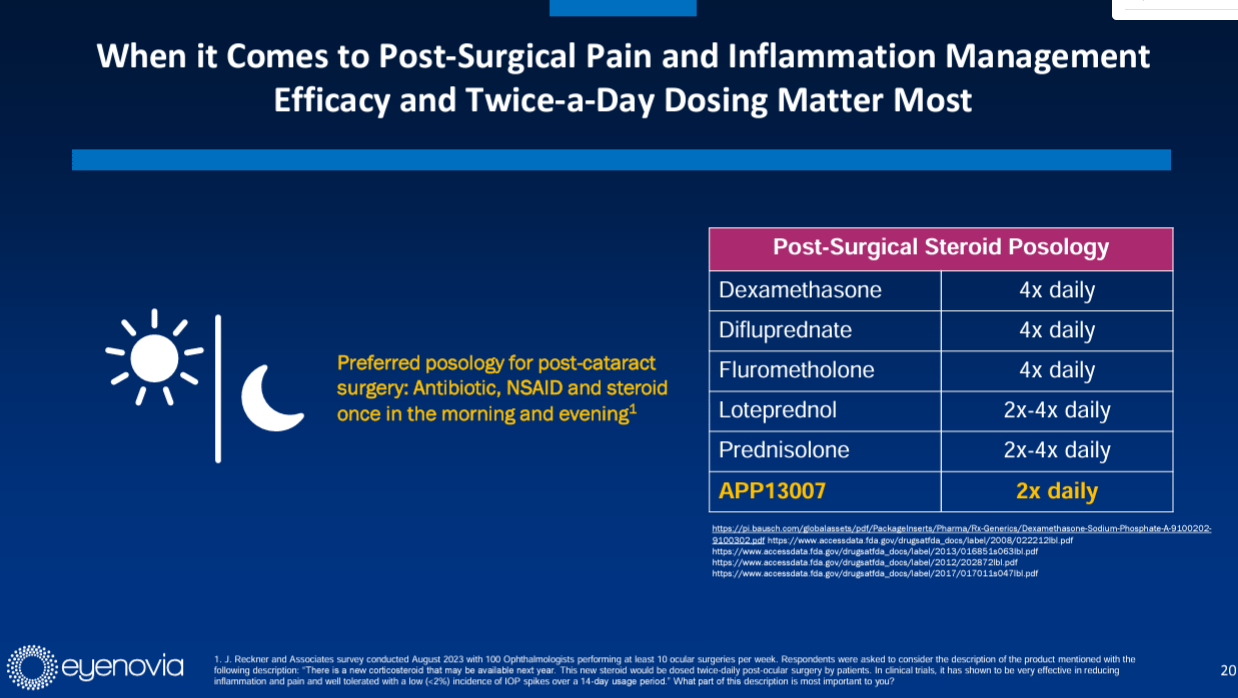
Drug’s superiority over existing treatments: stems from its preferred post-surgical steroid posology. APP13007 is proposed be dosed twice daily without titration, which is significantly more convenient than the 4 times a day dosing regimen with titration for existing treatments.
Market Potential: If approved, APP13007 will enter a ~$1.3-billion-dollar market for topical ophthalmic steroids and steroid combinations, driven by an estimated ~7 million ocular surgeries annually in the U.S. Eyenovia holds exclusive U.S. commercial rights to APP13007 as a potential treatment for post-surgical ocular pain and inflammation (obtained from Formosa Pharma).
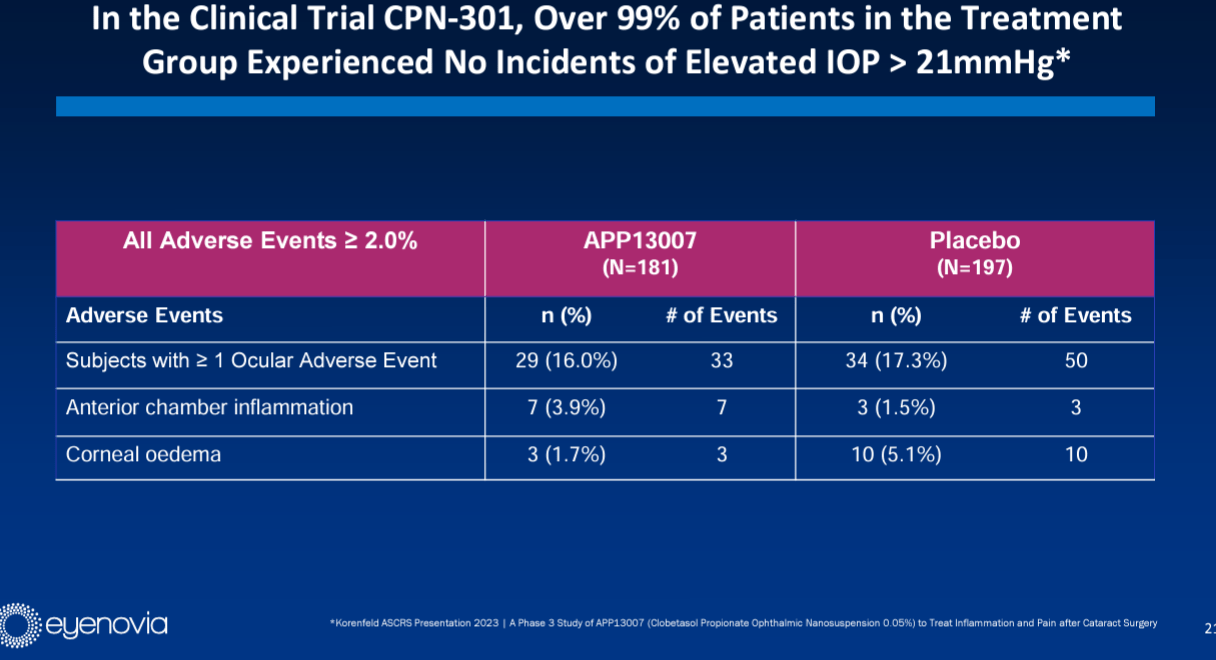
Upside optionality from dry eye indication: APP13007 has the potential for additional indications including dry eye in the Optejet (the company’s proprietary drug delivery device), a ~$3.6 billion market. When steroids are used for an extended time, the class of medications can cause an IOP spike, which is a transient intraocular pressure increase. This can lead to complications in patients with glaucoma. With APP13007, 99% of ~181 patients in a phase 3 trial saw no indications of elevated IOP rendering the profile favourable for its assessment as a dry eye treatment.
Revenues: The company is yet to generate any meaningful revenue, but that could change as it continues to focus on the commercialization of Mydcombi, its spray approved by the FDA for pupil dilation for eye examinations carried out before cataract surgery/corrective prescriptions. If APP13007 is also approved, it would complement Mydcombi, allowing for the generation of additional near-term revenue.
Management Guidance in Q3 earnings Call with respect to APP13007: ”This unique, twice-a-day steroid will compete against products that are typically used four times a day, with a desirable efficacy and safety profile. Together with Mydcombi, we expect the two products to increase the capability of our planned ten-person sales force to generate near-term revenue for our company which in turn will help fund the completion of our internal development programs.”


